SafeBreak® Vascular FDA Cleared for All Vascular Access Lines on Patients Two Weeks of Age and Up
04 Oct, 2023
#img0:hover{left:-242px;width:492px;height:305px}#img1:hover{left:-330px;width:580px;height:378px}#img2:hover{left:-306px;width:556px;height:353px}#img3:hover{left:-138px;width:388px;height:375px}#img4:hover{left:-105px;width:355px;height:374px}
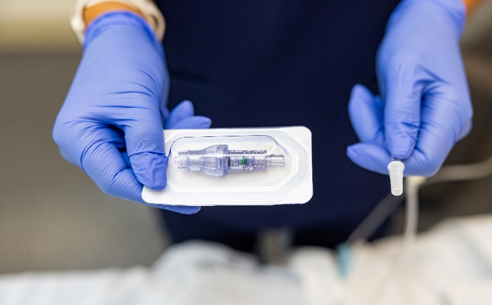
FAYETTEVILLE, Ark. – Oct. 3, 2023 – PRLog — Lineus Medical® is proud to announce the U.S. Food and Drug Administration (FDA) has cleared its flag ship product, SafeBreak® Vascular, for use on all vascular access lines including peripheral IV catheters, midlines, peripherally inserted central catheters, central venous catheters, IV ports, and interosseous vascular access devices on patients two weeks of age and up. This allows SafeBreak Vascular to be installed on both adults and children and on every type of IV line.
SafeBreak Vascular is the only breakaway device for IV lines proven to reduce IV complications.1 When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient’s IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. The separated device also causes the IV pump to alarm, notifying the nursing staff to check on the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patient’s infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1. It’s a win-win-win.
Ryan Brick, VP of Sales, stated, “This is a large milestone for our company and a steppingstone towards our mission of reducing IV-line failure. With SafeBreak’s clearance for all vascular lines, we can help protect every patient user and every IV line throughout the entire country. Sales momentum has continued to build since our pediatric clearance this past May, and now, the expansion of SafeBreak’s indications will bring even more interest and help introduce the product to more hospitals. We will continue to work to make SafeBreak the standard of care for every hospital and patient.”
Vance Clement, CEO of Lineus Medical, stated, “What an exciting time for SafeBreak Vascular and Lineus Medical! We recently gained licence in Canada and now FDA clearance for all medical lines for patients to two weeks of age and up in the United States. SafeBreak is a simple device that can make a big difference for nurses and hospitals. These new indications are for more complicated and more expensive IV lines that are used for long-term infusions, cancer patients, and in emergency situations. SafeBreak has been shown to work well on peripheral IVs, and now it can be used by nurses to protect these more expensive lines on more vulnerable patient populations. With these additional indications, we can expand the benefit of SafeBreak throughout the entire hospital.”
ABOUT LINEUS MEDICAL
Lineus Medical is the developer of a break-away technology that is proven to reduce IV restarts and IV complications1. Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found on the company website: www.lineusmed.com or you can follow the company on Facebook or LinkedIn.
1. Data on File
MKG-0237 Rev 00
Vance Clement
***@lineusmed.com
Photos:
https://www.prlog.org/12987006/1
https://www.prlog.org/12987006/2
https://www.prlog.org/12987006/3
https://www.prlog.org/12987006/4
https://www.prlog.org/12987006/5